WITLEAF Pulse Oximeters Pass Authoritative Global Validation, Earn Recognition from Open Oximetry Project
2025/06/06
Founded at UCSF’s Center for Health Equity in Surgery and Anesthesia (CHESA) and the UCSF Hypoxia Lab, the Open Oximetry Project has become one of the world's most authoritative pulse oximeter validation platforms. This project has rigorously tested devices from 32 leading global brands including Nellcor, Masimo, Nonin, and Edan, and received recognition from the the FDA, Center for Devices and Radiological Health (CDRH) in January 2024.
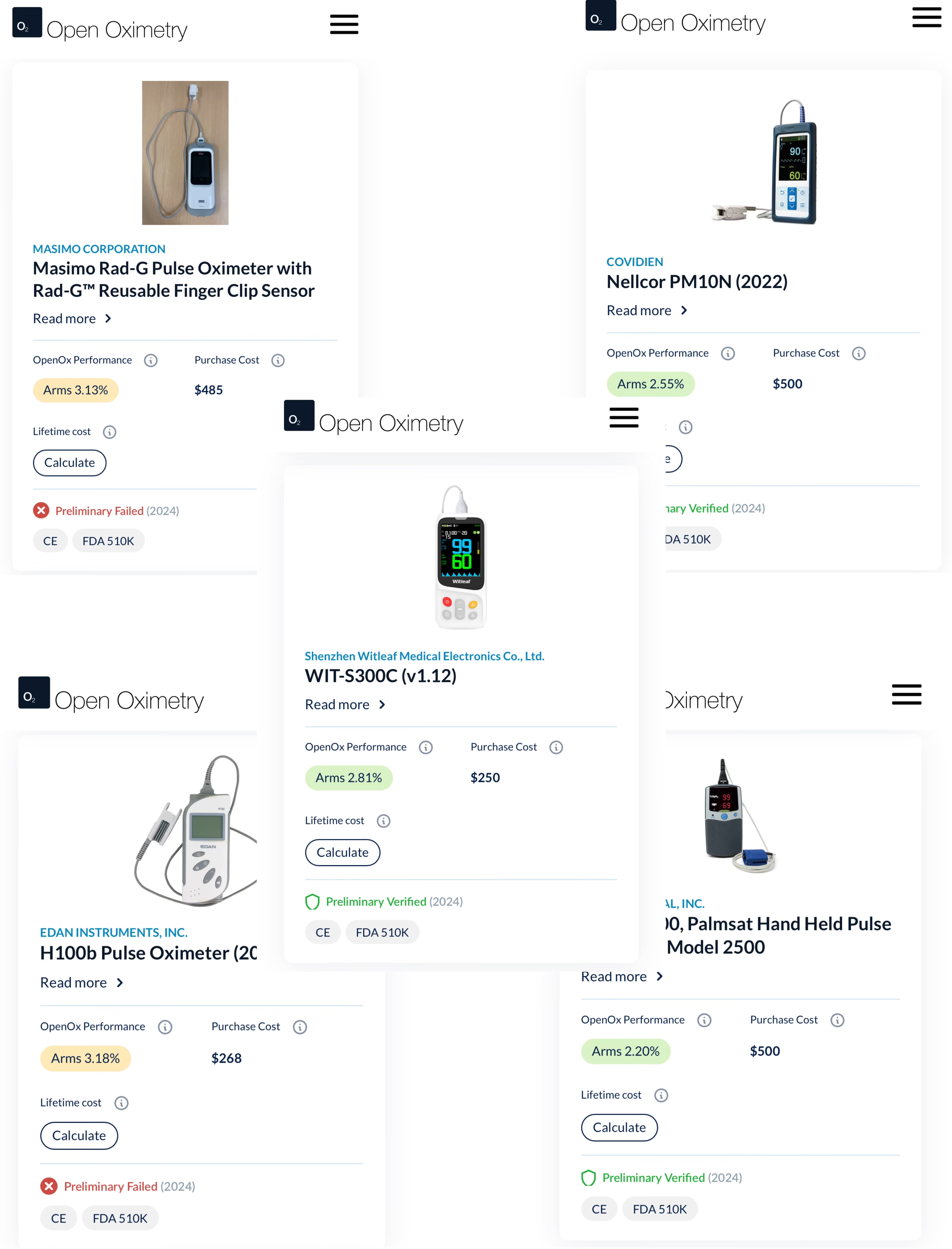
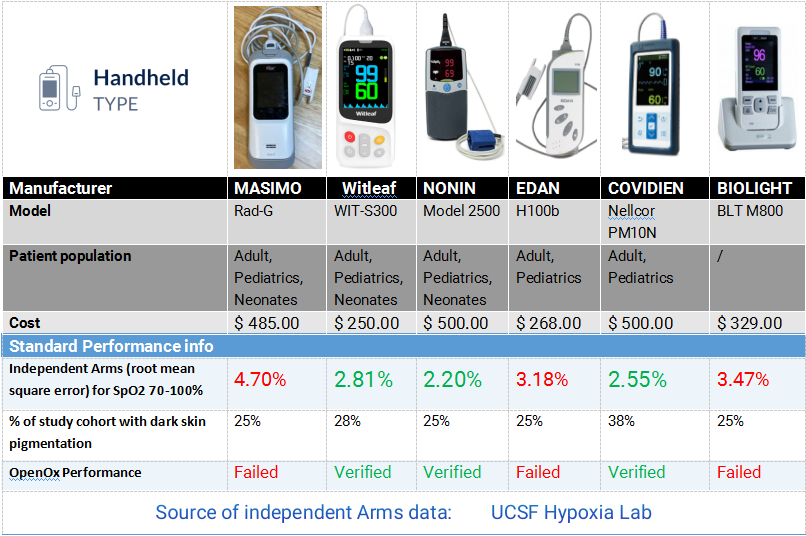
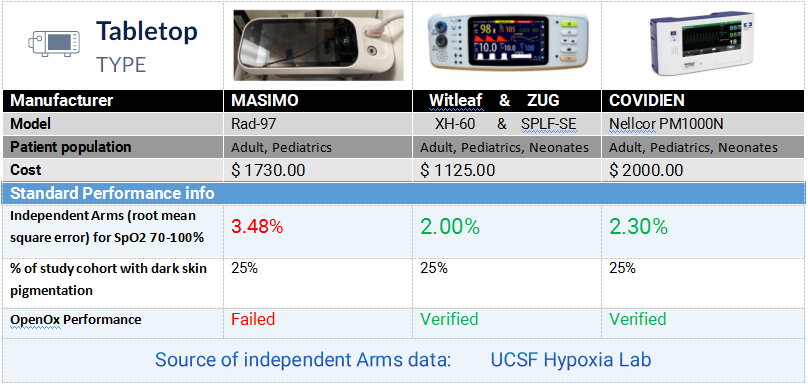
As one of the selected Chinese brands in this program, WITLEAF participated in the significant validation testing with two pulse oximeter models. The project followed internationally recognized clinical oximetry testing methods, where healthy subjects with varying skin tones inhaled controlled hypoxic gas mixtures under monitored conditions. For accuracy verification, arterial blood gas analysis - the clinical gold standard - was simultaneously performed and compared with the test devices' readings. The results showed WITLEAF oximeters delivered excellent performance, fully meeting both the FDA's 2013 Premarket Notification Guidance for pulse oximeters and the ISO 80601-2-61:2017 international standard requirements.

This certification not only validates WITLEAF products' excellence in accuracy, reliability and universality, but also demonstrates the brand's technical expertise in medical health monitoring. WITLEAF remains committed to providing global users with even more precise and reliable health monitoring solutions.
Open Oximetry Testing Report | Brand Device Performance Evaluation