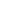
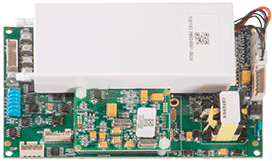
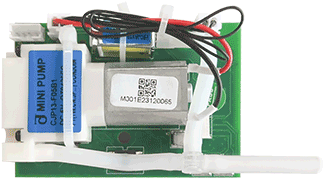
Technical Specifications
SPECIFICATIONS
VALUES
Parameters
NIBP,SPO2,PR,2*TEMP
Physical Dimensions
140mm×85mm×25mm
Voltage
DC 12V±5%
Power Consumption
≤6W (typical, at input voltage 12V±5%)
Communication Protocol
5V TTL-level UART interface
Operating Conditions
0°C to 70°C (32°F to 158°F),5%-95% non-condensing humidity
Storage Conditions
-40°C to 70°C (-40°F to +158°F),5%-95% non-condensing humidity
Advantage Technologies
Highly Integrated Multi-Parameter Modular Design
Advanced SpO₂ Monitoring, Even in Low-Perfusion Conditions
Dual-Domain SpO₂: Fuses time and frequency domains to suppress motion artifacts
Motion-Resistant NIBP Technology: multi-feature matching and frequency-domain interference rejection
Multi-Mode NIBP Measurement: Step deflation (AAMI-standard) and Inflation-Phase Monitoring
PWTT (Pulse Wave Transmission Time): Continuously tracks NIBP trends, minimizing measurement frequency
Self-established NIBP database covering hypertensive, elderly, neonatal and pregnant populations
OEM Customized Solutions
Please leave your information and we will contact you ASAP!
Witleaf invites you to fill in your personal information so that we can provide you with better service.
Related Products